
Zebrina detrita crawling on dew covered grass. Photographed last October near Kastamonu, Turkey.
Ever since I witnessed some land snails crawling around on dew covered plants one early and cold morning last October while I was in Turkey, I have gotten interested in the activities of snails and slugs at low temperatures.
I have also been reading about the fundamentals of dew formation. Nothing profound though, only the very basic stuff.
Dew is liquid water that condenses out of the air, usually at night, as a result of a drop in temperature. How does dew form?
Let's see if I got it right.
It's all thermodynamics and the thermodynamicists usually work with sealed systems constant at some property or other. So, consider a sealed vessel containing some water at a constant temperature, T1. Some of the water molecules in the liquid phase will evaporate into the vapor phase, while some of those in the vapor phase will return to the liquid. After a while, an equilibrium will be attained and after that point, the amount of water in the vapor phase (and in the liquid phase) will remain constant. The vapor phase will be saturated with water vapor. If we increase the temperature to T2, however, additional water will evaporate and at the new equilibrium point, there will be more water in the vapor phase than there was at T1.
Now, what will happen if we reverse the process and lower the temperature back to T1? Some of the water in the vapor phase will have to condense back into the liquid phase until the amount of water in the vapor phase returns to its equilibrium value at T1. As we see in the next diagram, the saturation amount of water in the vapor phase changes continuously as a function of temperature. Therefore, if the temperature of a system saturated with water vapor is lowered even just a tiny bit, some water will condense out.
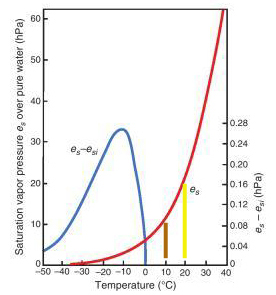
The red curve shows the variation with temperature of the saturation (equilibrium) vapor pressure of water. Ignore the blue curve. Fig. 3.9 from Atmospheric Science: An Introductory Survey by John Michael Wallace & Peter Victor Hobbs, Academic Press, 2006 (yellow and brown lines mine).
What if there isn't enough water in the vessel to saturate the vapor phase? In other words, if we put a very small amount of water into our vessel and the vapor phase is not saturated even after all of the water evaporates, will some of it still condense out when the temperature is lowered? Yes, it will if the temperature reaches a low enough value (less than T1) at which the vapor phase will be saturated with water.
This is basically how dew forms. If the air is saturated with water, in other words, when the relative humidity is 100%, the air is said to be at its dew point temperature (Td), because dew will be forming. If the air is not saturated, then the air temperature needs to fall down until the air becomes saturated with water vapor so that dew can start to form. That particular temperature will be the dew point temperature.
Let's look at the diagram again. Suppose the air temperature is 20 °C. The saturation pressure of water vapor at that temperature, marked by the yellow line, is ~20 hPa (hectopascals). But suppose that on a particular dry evening the vapor pressure happened to be ~10 hPa. That would make the relative humidity (10/20)x100=50%, which is the saturation vapor pressure at ~10 °C (brown line). Therefore, Td is 10 °C and dew will form if the air temperature drops down to 10 °C during the night.
So the dew point temperature is determined by 2 things: the temperature and the relative humidity.
There is a simple formula that gives the approximate Td if the relative humidity (RH) is >50%.
Td= T-((100-RH)/5)
In our example, Td= 20-((100-50)/5)=10. There are more exact dew point calculators here and here.
What does all of this have to do with snails?
In a
1. Snails need water to be active.
2. If during certain times of the year rains are infrequent, dew formation may be the only source of water available to snails.
3. If the daytime temperatures are in the range 14 to 20 °C and the relative humidity is low (~50%), then dew point temperatures will be much lower (<10 °C; see Figs. 1 & 2 in Lawrence, 2005).
4. Under such conditions, snails will be forced to be active at low temperatures.
This is exactly what I witnessed on that cold October morning.
Source
No comments:
Post a Comment